Research
Reactive Intermediates: The Key for Understanding Reactions
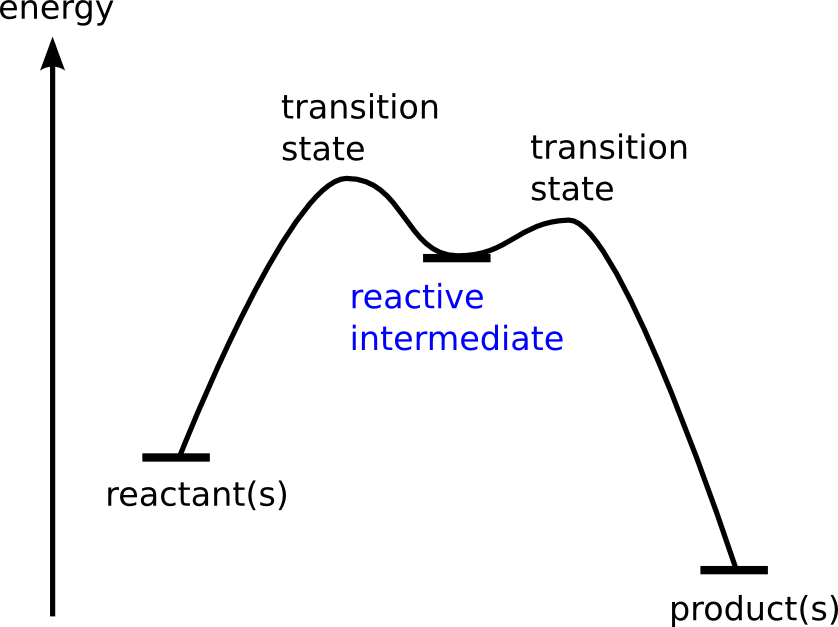
Chemical reactions pass through one or more transient stages – reactive intermediates – on their way from reactant to product. These short-lived reactive intermediates often determine the course of the reaction. Learning more about the fate and properties of reactive intermediates often brings not only a basic understanding of the elementary steps of a reaction, but also offers the possibility to steer the reaction.
Reactive intermediates play a key role in understanding the mechanistic details in homogeneous catalysis. We cannot only "see" and manipulate these important intermediates, but also understand reactivity based on recently developed novel methods to extract quantitative thermochemical and kinetic data.
Mass Spectrometry: Our Preferred Method for Studying Reactive Intermediates
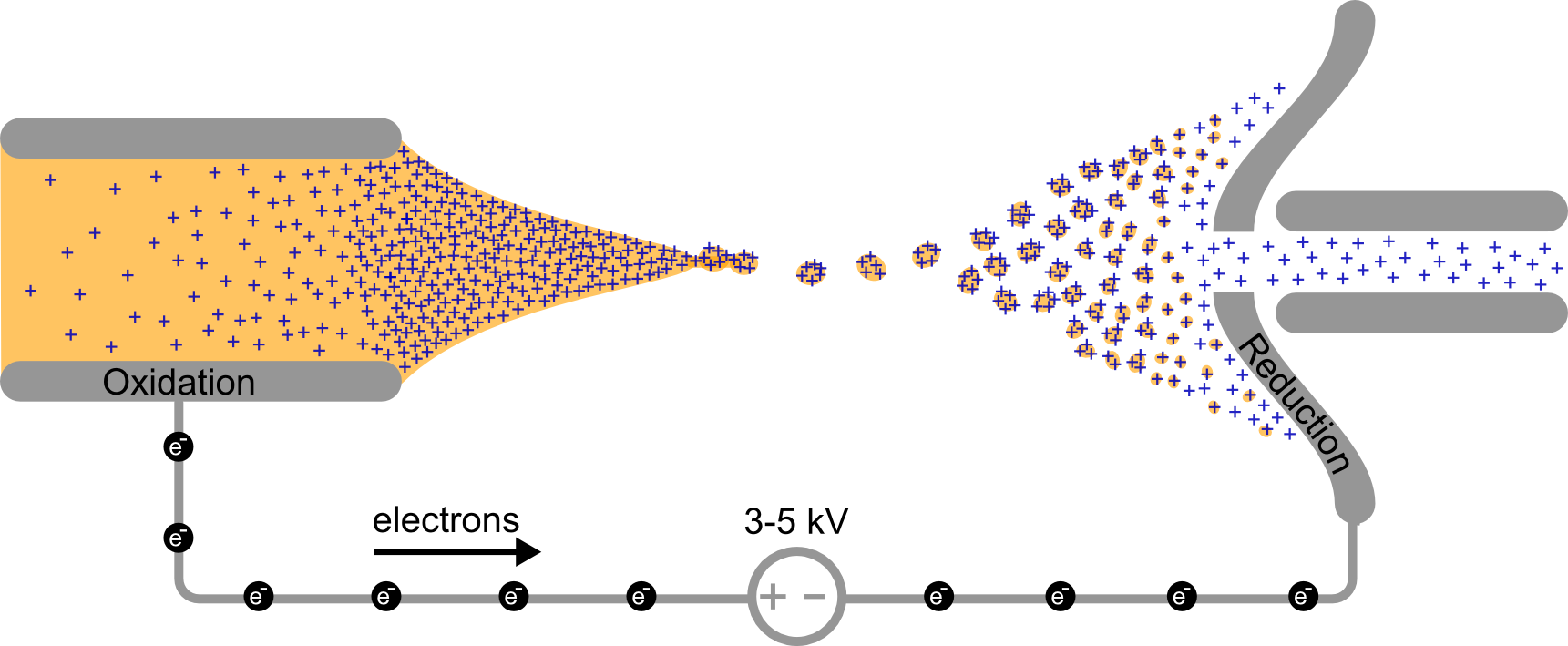
Reactive intermediates are short-lived and generally cannot be obtained as a solid or in high concentration. This makes the study by many spectroscopical techniques, such as IR, X-ray or NMR, impossible. Mass spectrometry (MS) on the contrary, allows for small concentrations and short lifetime of analytes.
Most reactive intermediates are not stable enough to survive even mild electrospray ionization (ESI) conditions. Therefore the reactive species are generated by suppling energy to precursor ions (e.g. by collisions) or by generating the reactive species under harsh spray conditions. ESI-MS requires charged analytes that preferably have as low number of fragmentation channels. It is therefore a privileged tool for investigating homogenous catalysis of metal complexes.
Information obtained by MS is free of solvent effects, which offers an unique opportunity to study these or to benchmark quantum chemistry.
Where mass spectrometry is not applicable, we study reactive intermediates indirectly, e.g. by kinetic experiments or by comprehending the relationship between structure and reactivity.
Thermochemistry from Gas-Phase Experiments
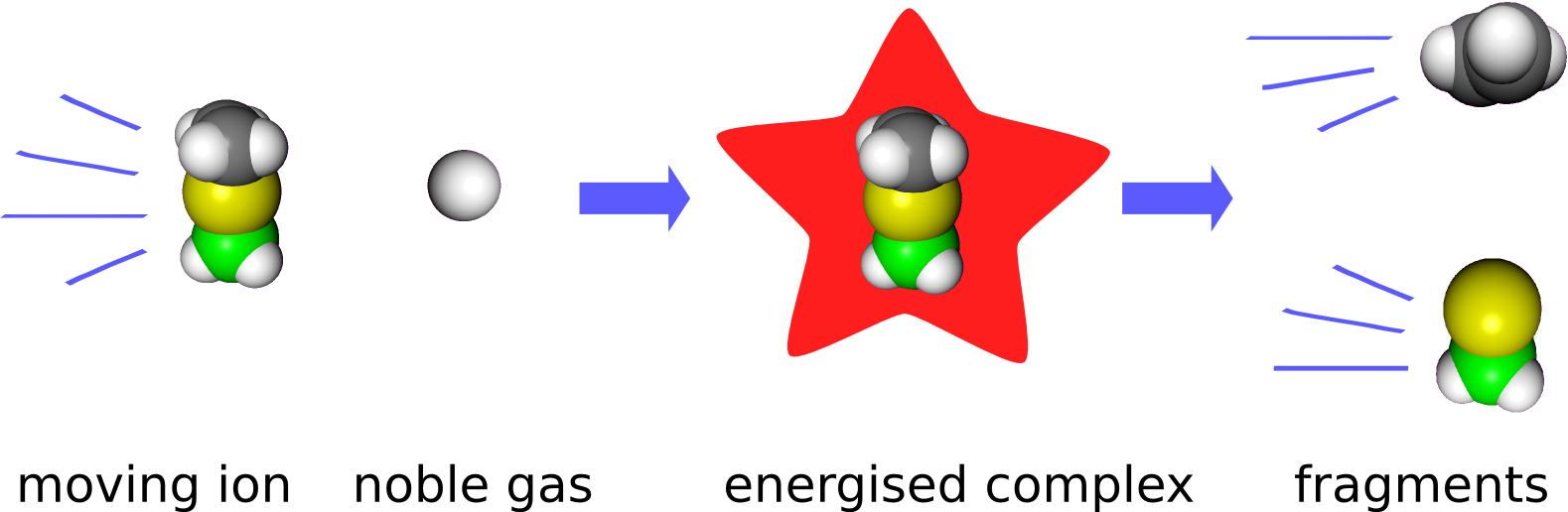
Thermodynamic data is preferably obtained from equilibrium measurements. For reactive intermediates, such measurements are per se impossible. Nonetheless, quantitative thermochemical data can be obtained from kinetic measurements. Normally, thermodynamics and kinetics of a reaction are should be kept well apart. However, in collision induced dissociation experiments (CID-experiments), there exists a theory that connects the two.
In CID-experiments, the ion of interest is accelerated by electrical fields in the mass spectrometer to a certain speed and then collided with a stationary noble gas atom. The kinetic energy of the ion is redistributed to rotations and vibrations, i.e. the ion is said to be energized. When the ion is energized enough, it fragments, which is easliy observable.
The better the energy content of the ions can be controlled, the more accurate the obtained thermochemical data is. For this reason we have customized a mass spectrometer. Data evaluation is conveniently handled by our own software, L-CID. Normally accuracies of ±1 kcal/mol are reached.