Reaction Development
Mechanism-Guided Reaction Design
The Chen group uses physical organic methods in solution, gas-phase measurements (both qualitative and quantitative) and computational chemistry to elucidate reaction mechanisms, usually catalyzed by transition metals. Spanning mechanistic work from classical kinetics in solution to isolating highly reactive and fleeting intermediates in the gas phase, these insights help us to rationally design new reactions or improve existing ones.
A recent focus has been on the study of (transition) metal carbenes and carbenoids as fleeting intermediates and their use in synthetic methodology.
Cyclopropanation
The two most often used methods for cyclopropanation, especially the simple methylenation, are the Simmons-Smith reaction and its derivatives as well as the transition-metal catalyzed decomposition of diazo compounds. Although these methods are versatile, they both suffer from their distinct drawbacks. Most notably the large amount of waste produced in the former and the hazardous nature of the reagent in the latter case.
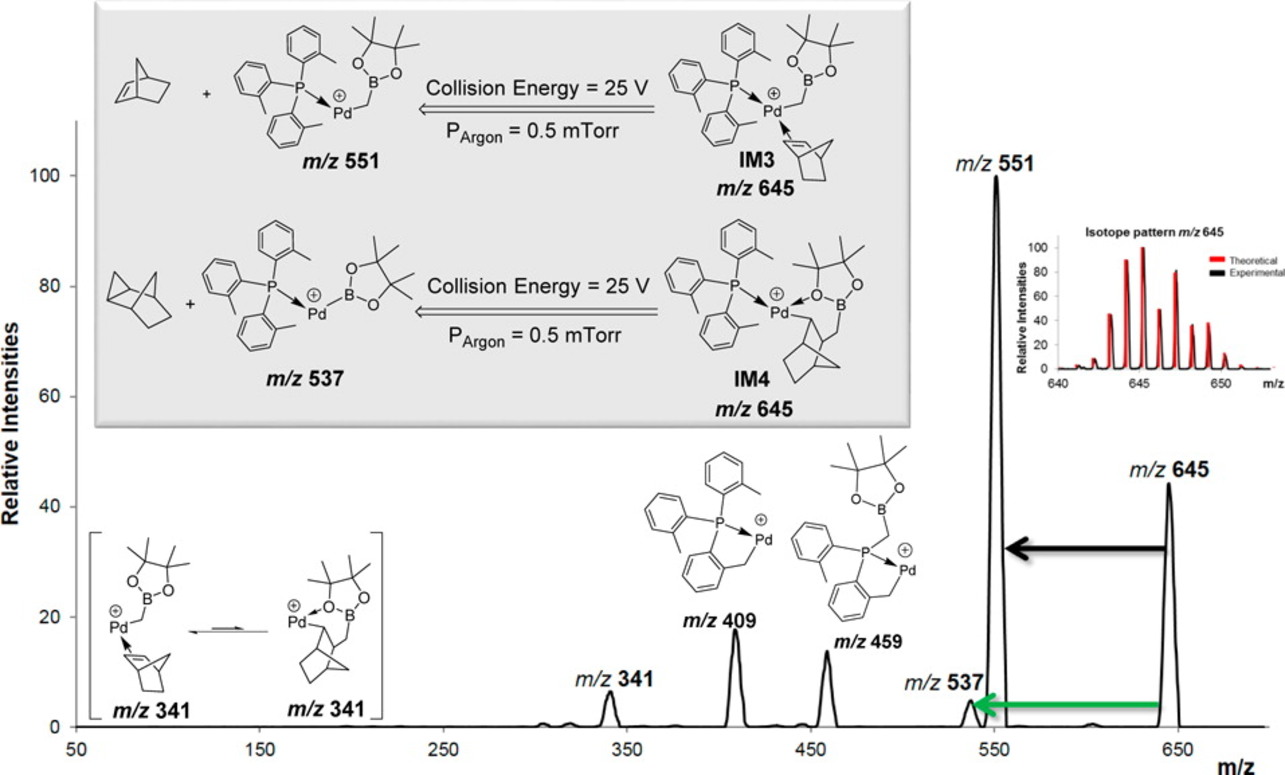
In order to circumvent these limitations, the group is involved in designing and improving alternative approaches and reagents. A recent example includes iodomethylboron compounds as a methylene donor in a ‘diverted Heck’ reaction.
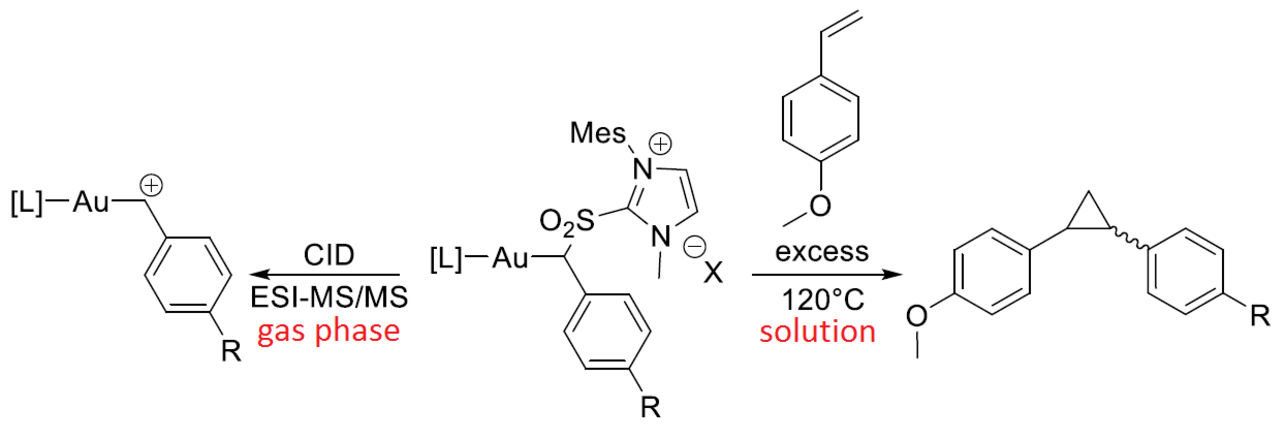
In another example a imidazolium sulfone was used as carbene precursor for a gold-catalyzed cyclopropanation reaction.
Metathesis
Homogeneous ruthenium complexes are a widely used class of catalysts for a variety of metathesis reactions, one example being the ring opening metathesis polymerization (ROMP) to form linear polymers from cyclic alkene precursors. The design of a dual-site catalyst allowed the sequence-selective co-polymerization of cyclooctene and norbornene.
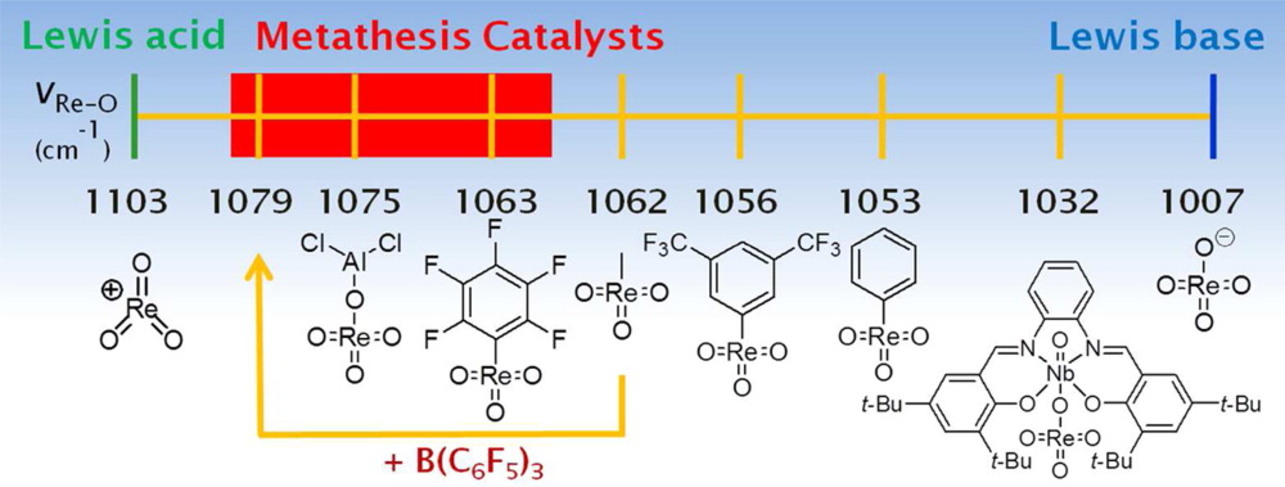
Heterogeneous catalysts based on rhenium (Re2O7/Al2O3, CH3ReO3/Al2O3-SiO2 or CH3ReO3/Nb2O5) are highly active and industrially important catalysts for olefin metathesis. Even today the exact nature of the active rhenium carbene and its formation are not entirely understood as the active species is only formed in situ.
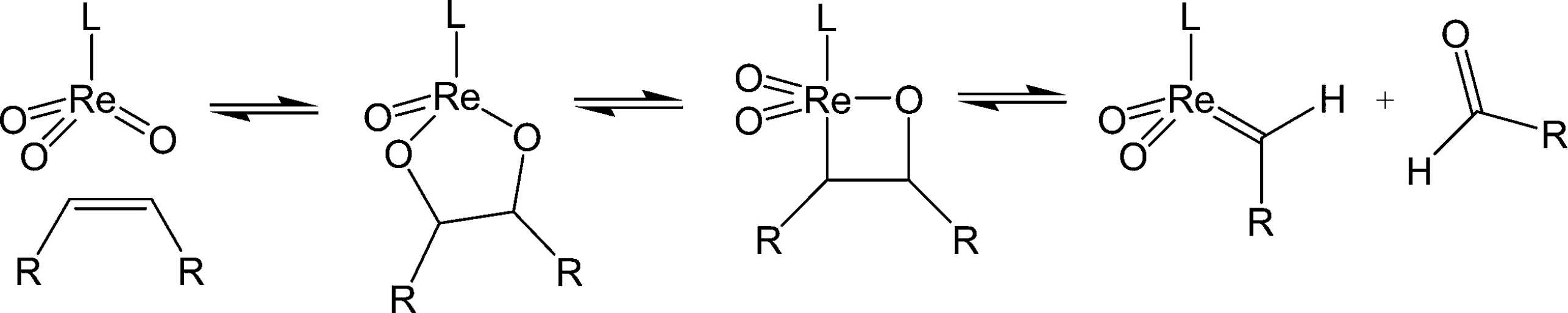
Gas-phase studies on homogeneous model compounds of high-valent trioxorhenium led to the postulation of a pseudo-Wittig mechanism as likely introduction into the catalytic cycle.
Various trioxorhenium model complexes have been synthesized, spanning the range between perrhenate (ReO4-) and perrhenyl (ReO3+), from which only the ones with certain electronic properties are active in olefin metathesis. It could be demonstrated that electron-withdrawing substituents or ligands, such as pentafluorophenyl or B(C6F5)3, are beneficial.