Dispersion Effect
London dispersion constitutes one of the fundamental interaction forces between atoms and between molecules. While modern computational methods have been developed to describe dispersive interactions in the gas phase properly, their importance in solution remains yet to be fully understood because experimental data is still sparse and current computationally affordable solvent models appear not to be adequate.
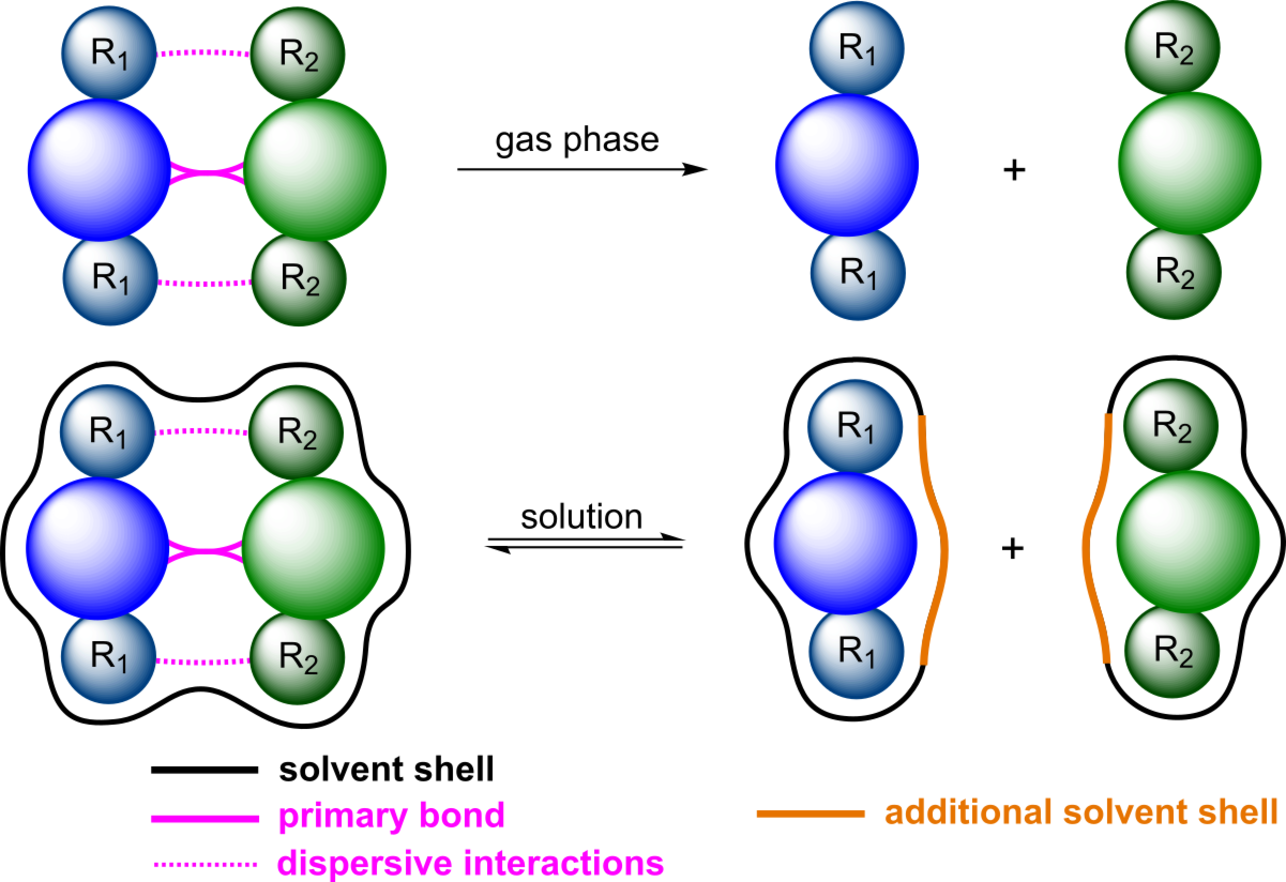
It is our goal to study dispersive interactions in carefully designed test systems in the gas phase and in solution both experimentally and computationally in order to build a solid data foundation. These test systems are designed to consist of two main fragments that are connected by a relatively weak central bond and the fragments carry substituents interacting with each other by dispersion. By investigating the bond dissociation of the central bond the corresponding influence of dispersion can be elucidated. From that, we seek to gain fundamental understanding of the importance of inter- and intramolecular London dispersion both in the gas phase and in solution and, ultimately, to rationally design and tune them.